UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
Relay Therapeutics, Inc. (the “Company”) will be conducting meetings with participants attending the 41st Annual J.P. Morgan Healthcare Conference (the “Conference”) during the week of January 9, 2023. A copy of the slides to be presented by the Company at the Conference is furnished as Exhibit 99.1 to this Current Report on Form 8-K, which is incorporated herein by reference.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Exhibits.
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
RELAY THERAPEUTICS, INC. |
|
|
|
|
Date: |
January 9, 2023 |
By: |
/s/ Brian Adams |
|
|
|
Brian Adams, J.D. |

JPM Presentation January 2023

Disclaimer This presentation contains forward-looking statements and information about our current and future prospects and our operations and financial results, which are based on currently available information. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ “opportunity,” ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include express or implied statements about the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates; the timing of disclosures regarding our pipeline and additional clinical data for RLY-4008 and initial clinical data for RLY-2608; the potential therapeutic benefits of our product candidates, including potential efficacy and tolerability, and combination potential of our product candidates; whether preliminary results from our preclinical or clinical trials will be predictive of the final results of the trials or any future clinical trials of our product candidates; the possibility that unconfirmed results from these trials will not be confirmed by additional data as the clinical trials progress; the competitive landscape and market opportunities for our product candidates; the expected strategic benefits under our collaborations; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; expectations regarding current and future interactions with the U.S. Food and Drug Administration (FDA); our ability to manufacture our product candidates in conformity with the FDA’s requirements; the capabilities and development of our DynamoTM platform; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and future clinical trials; our plans to develop, manufacture and commercialize our current product candidates and any future product candidates; and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of risks and uncertainties. These and other risks, uncertainties and important factors are described in the section entitled "Risk Factors" in our most recent Annual Report on Form 10-K or most recent Quarterly Report on Form 10-Q, as well as any subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent our views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners.

Relay Tx – Patient-Driven

Relay Tx – Dynamo™ Platform Dynamo™ Platform… …is focused on making medicines …aims to address selectivity on validated targets Modulation Hypothesis Hit Identification Lead Optimization Target Identification Develop-ment Commercial-ization Focus of platform PEOPLE Chemical biology insights Deep structural understanding EXPERIMENTATION Physics-based simulations AI / ML COMPUTATION 1 2 3 Selectivity Target Inhibition Tolerability Efficacy

Relay Tx – Execution Focused Public, clinical org Cash runway into 2025 5 disclosed programs 5+ unnamed programs Platform: + ML-DEL and Automation Presented clinical data at ESMO & Triple Meeting 3 assets in clinic Company Programs Jan 2020 Jan 2023 Private Preclinical Purely research 2 disclosed targets 6+ unnamed programs Company Programs Source: Relay Tx presentation at JPM conference Jan 2020

Target Program Annual US Patient # Breast Cancer1 PI3Kα franchise PI3KαPAN RLY-26082 ~8-51K ~50-156K all solid tumors RLY-58362 PI3KαSPECIFIC H1047R-specific ~4-25K ~15-48K all solid tumors Challengers CDK2 Selective CDK2 ~46K3 (Patients receiving CDK4/6i) Challengers Degrader ERα Degrader ~29-196K4 Undisclosed 1 program To be announced FGFR2 RLY-4008 Mutant + WT ~11-35K5 Tumor Agnostic SHP2 GDC-1971 ~37-69K6 Undisclosed 2 programs To be announced GD Genetic diseases 2 programs To be announced Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer Note: Unless otherwise indicated, patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs 1. Unless otherwise indicated, all breast cancer patient numbers refer to HR+/HER2- breast cancer tumors 2. RLY-2608 covers H1047X, E542X, E545X hot spots, and breast cancer patient range assumes HR+/HER2- population 3. ~46k HR+/HER2- breast cancer patients expected to receive CDK 4/6 inhibitors in adjuvant setting, first-line setting, and second-line setting in 2023, per Decision Resources Breast Cancer Market Forecast, report dated June 2022 4. HR+/HER2- US late-line breast cancer patients compared to HR+/HER2- US incident breast cancer patients 5. FGFR2 altered late-line solid tumors compared to comprehensive annual FGFR2 altered incident solid tumors including additional FGFR gene fusions and rearrangements resulting from truncation of the protein at exon 18 6. SHP2 combo only includes KRAS G12C in lung and CRC, EGFR mutations in lung, and ALK fusions in lung

Relay Tx – Capital, Team & Execution Focus to Deliver $1.1B Cash, cash equivalents and investments as of the end of 3Q 2022 Current cash, cash equivalents and investments are expected to be sufficient to fund current operating plan into 2025 Tumor Agnostic Breast Cancer Franchise GDC-1971 (SHP2) RLY-4008 (Selective FGFR2) Clinical start in early 2024 Selective CDK2 Ongoing combo trials; Genentech controls data disclosures PI3KαPAN Development candidate nomination in 2023 ERα Degrader Full dose escalation data in 1H 2023 Non-CCA expansion cohorts data in 2H 2023 Pivotal cohort full enrollment in 2H 2023 Initial data in 1H 2023 To be announced 5+ undisclosed programs in preclinical development and additional early-stage efforts across platform Undisclosed RLY-2608 Clinical start in 2Q 2023 RLY-5836

Relay Tx Programs

Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 Mutant + WT Tumor Agnostic SHP2 GDC-1971 Undisclosed 2 programs GD Genetic diseases 2 programs Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer

FGFR2 – Validated Target Present in Several Tumor Types Fusions3 Amplifications Mutations FGFR2 Annual Cases (US, comprehensive) FGFR2-altered cancers remain a high unmet medical need Pancreatic Breast Endometrial Skin Melanoma Stomach Lung Bladder CUP Ovarian ~5K-15K ~4.5K-7.5K FGFR2 alterations are observed across multiple tumor types2 Three classes of driver alterations in FGFR2 Cholangio Annual US Patient Count1 Total FGFR2 alterations1: ~10-23K patients 36-42% Objective Response Rate4 FDA approvals only in fusion+ CCA FGFRi-naïve patients Limited treatment options for other FGFR2 driven cancers5 Sources: Image adapted from Babina IS, Turner NC. Nat Rev Cancer 2017;17: 318-332; Internal analysis based on third party industry data 1. All patient #’s refer to total annual number of US patients with late-line cancers vs. comprehensive annual incidence that may be amenable to treatment with our programs including additional FGFR gene fusions and rearrangements resulting from truncation of the protein at exon 18; 2. Cholangio, cholangiocarcinoma (CCA); CUP, carcinoma unknown primary; 3. FGFR2 fusion estimates include del18 truncations; 4. Based on pemigatinib, erdafitinib, and futibatinib prescribing information; 5. Erdafitinib is approved for urothelial carcinoma with FGFR2/3 alterations

FGFR2 – Limitations of Current FGFR Inhibitor Landscape Sources: Pemigatinib – prescribing information; futibatinib – prescribing Information; erdafitinib – prescribing information 1. From pemigatinib NDA review documents: "Pemigatinib 13.5 mg daily provided 76% inhibition of ex vivo phosphorylated FGFR2α at trough" FDA Approved Compound % of Patients with Hyperphosphatemia % of Patients with Diarrhea Pemigatinib 94% 47% Futibatinib 88% 39% Erdafitinib 76% 47% Limited Tolerability Limited Target Inhibition Limited Efficacy 36-42% Objective Response Rate in Fusion+ CCA FGFRi-naïve pts Limited Selectivity Approved Pan-FGFRis are non-specific across FGFR family Pemigatinib 13.5mg QD achieves 76% inhibition of FGFR2 at trough1 High rates of off-target toxicity (esp. FGFR1,4) Limited Limited Limited Limited

FGFR2 – Standard Approach to Discovery Has Had Limited Success Standard Approach FGFR1 FGFR2

FGFR2 – Increasing Resolution Reveals New Opportunities FGFR1 FGFR2 Down Up Down Up Down Up Down Up Down Up Up Up Down Down Down Up Exploiting the dynamic difference between FGFR1 and FGFR2 enabled Relay Tx to design a selective FGFR2 inhibitor We predicted that a segment of FGFR1 would be fully extended outwards more frequently than the same segment in FGFR2

RLY-4008 – Is A Highly Selective and Irreversible Inhibitor Pan-FGFR Inhibitors RLY-4008 AZD4547 Erdafitinib Pemigatinib Futibatinib FGFR2 Note: Single experiment that tested each compound run at 500nM against 468 targets in the absence of ATP and without preincubation Source: KINOMEscanTM by Eurofins DiscoverX FGFR1 FGFR2 FGFR3 FGFR4 Percent Control Percent Control

RLY-4008 – ReFocus Trial Design Part 1: Dose Escalation Unresectable or metastatic solid tumors FGFR2 alterations per local assessment Both FGFRi-naïve & FGFRi-treated allowed RLY-4008 RP2D: 70 mg QD Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments Data from 2022 ESMO Congress (September 2022)

RLY-4008 – Patient Characteristics Fusion+ CCA FGFRi-Naïve1 Total (N=195)2 Parameter 70 mg QD (N=17) All doses (N=38) Age (years), median (range) 57 (36-81) 58 (33-81) 59 (23-87) Female, % 59% 58% 62% Race, % White / Asian / Black / Unknown 41% / 24% / 0% / 35% 58% / 21% / 3% / 18% 63% / 15% / 4% / 18% ECOG PS3, % 0 53% 50% 38% 1 47% 50% 58% 2 0% 0% 3% Prior lines of systemic therapy, % 0 0% 0% 2% 1 41% 47% 20% 2 47% 32% 29% 3+ 12% 21% 49% Baseline sum of target lesions (RECIST 1.1, mm), median (range) 57 (10-157) 63 (10-216) 79 (10-274) Efficacy analysis includes patients with previously treated, FGFR2i-naïve CCA treated at the RP2D. Patients with measurable disease who had opportunity for ≥2 tumor assessments to confirm response or discontinued treatment with <2 tumor assessments Patients in safety population who received ≥1 dose of RLY-4008 at any dose level ECOG PS = Eastern Cooperative Oncology Group Performance Scale Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments Data from 2022 ESMO Congress (September 2022)

Approved Pan-FGFR Inhibitors Demonstrate 23-36% ORR in This Population3 RLY-4008 – Interim Response Data FGFRi-Naïve Fusion+ CCA Patients at Pivotal Dose (70 mg QD) Best RECIST Change (%) from Baseline Based on Investigator Evaluated Response for Fusion+ CCA FGFRi-Naïve Patients at 70 mg QD (N=17) 1. Confirmed ORR = 82%: 14 confirmed PRs, 1 unconfirmed PR in an ongoing patient; 2. Complete tumor resection: R0 resection performed in curative intent, patient remains no evidence of disease as of 31 May 2022; 3. Referenced approved pan-FGFRi are Pemigatinib and Infigratinib; ORR based on prescribing information. These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments SD uPR PR Stable Disease Unconfirmed Partial Response Confirmed Partial Response Progressive Disease PD BOR = Best Overall Response: 88% ORR (15 of 17 patients)1 Went on to Resection with Curative Intent2 Data from 2022 ESMO Congress (September 2022)

RLY-4008 – Interim Response Data FGFRi-Naïve Fusion+ CCA Patients Across All Doses 92% of Patients With Tumor Reduction Across All Dose Levels, Majority of Patients With Partial Response per RECIST 1.1 Best RECIST Change (%) from Baseline Based on Investigator Evaluated Response for Fusion+ CCA FGFRi-Naïve Patients Across All Doses (N=38) Confirmed ORR = 58%: 22 confirmed PRs, 2 unconfirmed PR Complete tumor resection: R0 resection performed in curative intent, patient remains no evidence of disease as of 31 May 2022 Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments Went on to Resection with Curative Intent2 SD uPR PR Stable Disease Unconfirmed Partial Response Confirmed Partial Response Progressive Disease PD BOR = Best Overall Response: 63% ORR (24 of 38 patients)1 Data from 2022 ESMO Congress (September 2022) QDi = once daily dosing on an intermittent schedule (3 weeks on drug, 1 week off); BID = twice daily dosing

RLY-4008 – Time on Treatment for Fusion+ CCA FGFRi-Naïve Patients (All Doses) Complete tumor resection: R0 resection performed in curative intent, patient remains no evidence of disease as of 31 May 2022 Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments SD uPR PR Stable Disease Unconfirmed Partial Response Confirmed Partial Response Progressive Disease PD BOR = Best Overall Response: 13 of 15 (87%) 70 mg QD responders remain on treatment Complete Tumor Resection1 Duration of exposure (weeks) Median duration of exposure: 5.5 months (range: <0.1 to 18.5 months) Median time to response: 1.8 months 12/38 (32%) Discontinued - 1 resection with curative intent, 8 PD, 1 AE, 2 withdrawal of consent Went on to Resection with Curative Intent1 Data from 2022 ESMO Congress (September 2022)

RLY-4008 – Treatment-Related Adverse Events (TRAEs) Interim Profile TRAEs > 15% TRAE Dose Modification RP2D, 70 mg QD (N=89) All Doses (N=195) Dose interruption (%) 42% 47% Dose reduction (%) 27% 33% Dose discontinuation (%) 1% 1%* Clinically Insignificant Off-Target Hyperphosphatemia (12%, all Gr 1-2) and Diarrhea (4%, all Gr 1-2) Allow for Optimization of FGFR2 Inhibition Doses at ≥40 mg QD result in 90%+ target inhibition Most AEs have been expected FGFR2-on target, low-grade, monitorable, manageable and largely reversible RP2D, 70 mg QD (N=89) All doses (N=195) Data from 2022 ESMO Congress (September 2022) All Grades Grade 3 * 1 hypersensitivity, 1 retinal pigment epithelial detachment, both resolved Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments

RLY-4008 Poised for Tumor Agnostic Validation Across FGFR2 Alterations Non-CCA advanced, solid tumors with FGFR2 alterations Non-CCA patients with FGFR2-fusion Non-CCA patients with FGFR2-amplification Non-CCA patients with FGFR2-mutation Tumor regression observed across FGFR2 mutations and amplifications in ReFocus Part 1 Dose Escalation Data Mutations (N=7) Amplifications (N=3) BC BC BC BC Breast Cancer BC Continue to actively enroll tumor agnostic cohorts Data presented at 2021 ENA Meeting (data as of 09 September 2021) Data from 2021 Triple Meeting (October 2021, N=49 patients) Data Disclosure From Tumor Agnostic Cohorts Anticipated in 2023

Relay Tx Solution – Addressing Unmet Need Through Greater Selectivity Sources: KINOMEscanTM by Eurofins DiscoverX; RLY-4008 data as presented at ESMO Congress 2022 1. Interim data as of 01 August 2022; 2. Single experiment that tested each compound run at 500nM against 468 targets in the absence of ATP and without preincubation; 3. Toxicity rates across all doses, n=195 patients Favorable Selectivity1 ~200x selective for FGFR2 over FGFR1, ~5000x selective over FGFR42 Favorable Target Inhibition1 FGFR2 Favorable Interim Efficacy1 Doses at ≥40 mg QD result in 90%+ target inhibition Favorable Interim Tolerability1 Minimized key off-target toxicities3 Hyper-phosphatemia1 Diarrhea Discontinuation 12% 4% 1% All Gr1-2 All Gr1-2 FGFR2 driven tumor shrinkage: 88% ORR in fusion+, FGFRi-naïve CCA 15 of 17 pts at 70mg QD pivotal dose (based on interim data) Most AEs have been expected FGFR2-on target, low-grade, monitorable, manageable and largely reversible Favorable Favorable Favorable Favorable 95% RO 90% RO RLY-4008 Concentration (Mean+SD) (ng/mL) Time (hr) 63% interim ORR for fusion+, FGFRi-naïve CCA across all doses

Part 1: Dose Escalation Demonstrate clinical proof of mechanism RLY-4008 – ReFocus Trial RP2D Target Inhibition Tolerability Selectivity Part 2: Dose Expansion and Pivotal Cohort Confirm dose and establish efficacy Other FGFR2-altered CCA Pivotal: Fusion+ CCA, FGFRi-naïve FGFR2-altered, non-CCA tumors Advanced solid tumors with FGFR2 alterations per local assessment; Both FGFRi-naïve & FGFRi-treated allowed Focus of Disclosure Early Signals of Efficacy 4Q 2021: Triple Meeting Validated hypothesis with target inhibition and acute tolerability Provided early efficacy signals in heterogeneous populations 2023: Anticipated Milestones 1H 2023: Full dose escalation data 2H 2023: Pivotal cohort full enrollment 2H 2023: Non-CCA expansion cohorts data 3Q 2022: ESMO Confirmed longer term safety and tolerability Established stronger support for FGFR2 fusion+ CCA, FGFRi-naïve efficacy Interpretable Efficacy

Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 – Mutant + WT Tumor Agnostic SHP2 GDC-1971 Undisclosed 2 programs GD Genetic diseases 2 programs Relay Tx – Emerging Breast Cancer Franchise Preclinical Early Clinical Late Clinical CCA + other Breast Cancer

Breast Cancer – Limitations of Current Standard of Care …is limited by efficacy of available treatments HR+/HER2- breast cancer standard of care1… Adjuvant 1L 2L+ Chemo Chemo Chemo / Enhertu® Targeted Tx Targeted Tx Targeted Tx Endocrine Tx (AI, SERD2) CDK4/6 +/- Endocrine Tx (AI, SERD2) CDK4/6 +/- ET2, CDK4/6, PI3K, mTOR ~200k annual HR+/HER2- breast cancer patients in US, of whom ~60k advance to later lines of treatment Source: Internal analysis based on third party industry data 1. Standard of care for HR+/HER2- breast cancer is illustrative; 2. AI = Aromatase Inhibitor; SERD: Selective Estrogen Receptor Degrader; ET = Endocrine Therapy Efficacy Selectivity Target Inhibition Tolerability Limited Limited Limited Limited

Relay Tx Solution – Highly Selective Breast Cancer Franchise Aspirational future state standard of care (HR+/HER2- BC)1 Targeted Tx Chemo / Enhertu® Chemo Chemo Adjuvant 1L 2L+ Targeted Tx Targeted Tx Next Gen Endocrine Tx Selective CDKs Others Mutant PI3Kα Next Gen Endocrine Tx Selective CDKs Mutant PI3Kα Next-gen ET Sel. CDKs Mut. PI3Kα Relay Tx aims to transform the standard of care for HR+/HER2- breast cancer Relay Tx Breast Cancer Portfolio PI3Kα Franchise RLY-2608 (pan-mutant) RLY-5836 (pan-mutant) H1047R (mutant-specific) Selective CDK2 Inhibitor ERα Degrader Other Undisclosed Programs Relay Tx Solution 1. Aspirational future state standard of care for HR+/HER2- breast cancer is illustrative

Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 Mutant + WT Tumor Agnostic SHP2 GDC-1971 Undisclosed 2 programs GD Genetic diseases 2 programs Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer
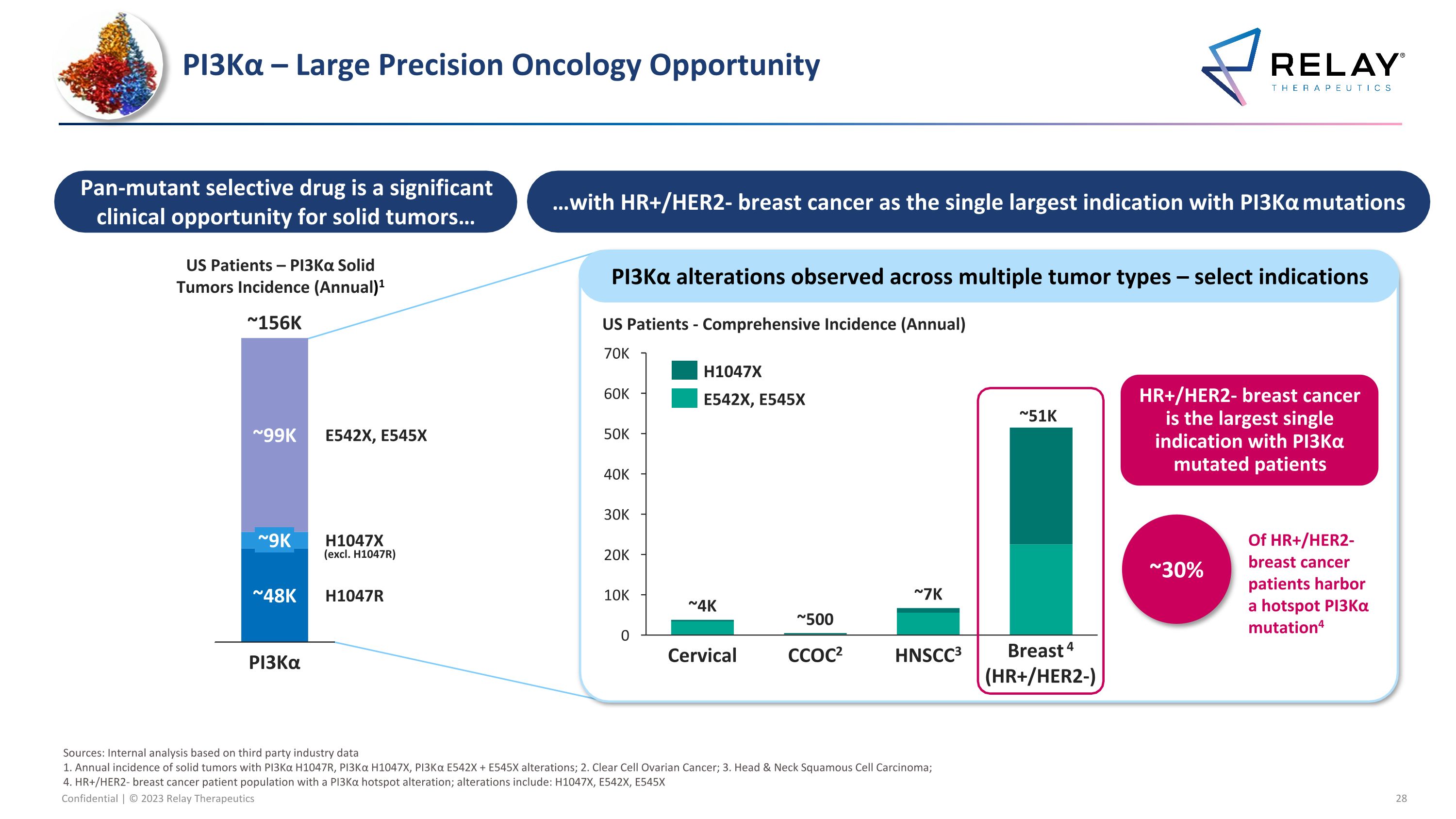
PI3Kα – Large Precision Oncology Opportunity (excl. H1047R) HR+/HER2- breast cancer is the largest single indication with PI3Kα mutated patients Pan-mutant selective drug is a significant clinical opportunity for solid tumors… …with HR+/HER2- breast cancer as the single largest indication with PI3Kα mutations ~30% Of HR+/HER2- breast cancer patients harbor a hotspot PI3Kα mutation4 PI3Kα alterations observed across multiple tumor types – select indications 50K 40K 0 20K 10K 30K 60K 70K ~500 Breast 4 (HR+/HER2-) Cervical CCOC2 HNSCC3 ~4K ~7K ~51K H1047X E542X, E545X US Patients - Comprehensive Incidence (Annual) Sources: Internal analysis based on third party industry data 1. Annual incidence of solid tumors with PI3Kα H1047R, PI3Kα H1047X, PI3Kα E542X + E545X alterations; 2. Clear Cell Ovarian Cancer; 3. Head & Neck Squamous Cell Carcinoma; 4. HR+/HER2- breast cancer patient population with a PI3Kα hotspot alteration; alterations include: H1047X, E542X, E545X H1047R ~99K ~9K ~48K E542X, E545X PI3Kα H1047X ~156K US Patients – PI3Kα Solid Tumors Incidence (Annual)1

based PI3Kα – Existing Inhibitors Have Limited Therapeutic Window Limited Target Inhibition Limited Tolerability Compound All Gr3+ Tox Hyperglycemia GI Tox (all Gr) Rash (all Gr) All Gr Gr3+ Alpelisib1-7 44-78% 33-65% 13-37% 33-60% 20-36% Inavolisib8-12 33-54% 55-70% 5-22% 27-50% 7-27% Note: fulv = fulvestrant; BC= breast cancer; all referenced studies are for their patient populations which are analogous to ongoing patient populations within RLY-2608 clinical trials; Alpelisib and fulvestrant are FDA approved, Inavolisib is in Phase 3 clinical trials Sources: Alpelisib – 1. SOLAR-1: Andre 2019 N Engl J Med 380:1929, 2. Ph 1b: SABCS 2013 P2-16-14, 3. Ph 1b: SABCS 2014 PD5-5, 4. Ph 2 ByLIEVE: Rugo 2021 Lancet Oncol 22:489, SABCS 2021 #P1-18-03, 5. Ph 1b mono: Annals of Oncol 25 2014 (suppl 4), 6. Ph 2 mono: Savas Cancer Discov 2022 Sep 12:2058, 7. Ph 1a mono: Juric 2018 J Clin Oncol 36:1291; Inavolisib – 8. ASCO 2022 #1052, 9. SABCS 2020 #PS11-11, 10. AACR 2020 CT109, 11. SABCS 2019 OT1-08-04; 12. SABCS 2019 P1-19-46, 13. SABCS 2021 #P5-17-05; Inavolisib + fulvestrant Ph 1b13 Alpelisib + fulvestrant Ph 24 Alpelisib Monotherapy Ph 1a7 Limited Efficacy 6% 19% 19% ORR ORR ORR Limited Selectivity Potency (nM) H1047R E542K E545K WT Alpelisib H1047R E542K E545K WT Alpelisib and inavolisib are equipotent for WT and mutant PIK3α Both also retain nM potency against other PI3K isoforms Inavolisib Limited Limited Limited Limited Regimen Interruption Reduction Discont. Alpelisib6,7 58% 38% 15% Alpelisib + fulv1 74% 64% 25% Inavolisib + fulv8 41% 18% 2% Alpelisib: Observed coverage (based on IC80) at average clinical dose 9-13hr7

PI3Kα – Proprietary Insights Unlock Novel Approaches A differentiated understanding of the structure of PI3Kα and its relationship to function equips Relay Tx to design optimal mutant-selective inhibitors of PI3Kα Designed pan-mutant selective PI3Kα inhibitor (PI3KαPAN) Discovered novel allosteric pocket favored in mutant protein Solved first full-length structures of PI3Kα (mutant and wild-type) Orthosteric Site Mutant PI3Kα

RLY-2608 – Mutant and Isoform Selectivity Potency (nM) H1047R E542K E545K WT Alpelisib Inavolisib RLY-2608 RLY-2608 has shown biochemical selectivity for mutant over wild type PI3Kα α-mut Potency (nM) β δ Alpelisib RLY-2608 Inavolisib RLY-2608 was inactive on other isoforms γ α-mut β δ γ α-mut β δ γ PI3K Isoforms Mutant vs. WT PI3Kα potency Mutant PI3Kα vs. other isoform potency H1047R E542K E545K WT H1047R E542K E545K WT δ (delta) isoform is an important anti-target for GI toxicity Source: RLY-2608 data as presented in 2021 AACR-NCI-EORTC Molecular Targets Conference poster presentation Selectivity

RLY-2608 – Selective Across the Kinome RLY-2608 inhibited only PI3Kα, with preferential inhibition of mutant Source: RLY-2608 data as presented in 2021 AACR-NCI-EORTC Molecular Targets Conference poster presentation Selectivity

In higher species, dosing of RLY-2608 for 28 days showed no histopathological or ophthalmic findings associated with hyperglycemia RLY-2608 – Reduced Impact on Glucose Homeostasis Tolerability Hyperglycemia Definitions (CTCAE v5.0) Grade CTCAE Definition (v5.0) Gr 1 Abnormal glucose above baseline, no medical intervention Gr 2 Change in daily management from baseline for diabetic; oral antiglycemic agent initiated; workup for diabetes Gr 3 Hospitilization indicated; insulin therapy initiated Gr 4 Life-threatening consequences; urgent intervention indicated Gr 5 Death Source: NIH, Common Terminology Criteria for Adverse Events (CTCAE) version 5.0

RLY-2608 – In Vivo Tumor Regressions Target Inhibition and Efficacy

Limited potency against WT PI3Kα and other PI3K isoforms RLY-2608 – First Mutant Selective Inhibitor to Enter the Clinic Potency (nM) Alpelisib Inavolisib RLY-2608 WT Mutant WT Mutant WT Mutant Favorable Selectivity Favorable Efficacy Favorable Target Inhibition Manageable key toxicities, especially hyperglycemia shown in dog study Favorable Tolerability All Data Shown is Preclinical Favorable Favorable Favorable Favorable Robust tumor regression at tolerable doses in mouse model Maintains approx. 80% mutant PI3Kα inhibition in mouse model

RLY-2608 – Trial Design Part 1: Dose Escalation 1. Excludes PIK3CAmut clear cell OvCA, HNSCC, and Cervical cancer patients; 2. Double mutation defined as one major PIK3CA mutation (E542X, E545X, H1047X) + ≥1 additional PI3KCA mutation per local assessment; 3. Intolerance to PI3Kα inhibitors is defined as treatment discontinuation due to treatment-related AE (e.g., hyperglycemia, rash, diarrhea, stomatitis) other than severe hypersensitivity reaction and/or life-threatening reactions, such as anaphylaxis and Stevens-Johnson syndrome. Part 2: Dose Expansion Initial clinical data update expected in 1H 2023 Part 3: Pivotal Trial PIK3CAmut advanced solid tumors PIK3CA double mutant adv. solid tumors2 (N=15) PIK3CAmut Clear Cell OvCA (N = 15) PIK3CAmut HNSCC (N = 15) PIK3CAmut Cervical CA (N = 15) Other solid tumors with a PIK3CAmut1 (N = 15) MTD/RP2D RLY-2608 Arm Includes mixed histologies Registration path to be determined PIK3CAmut, HR+, HER2- advanced breast cancer, with NO prior PI3Kα inhibitor (N = 15) PIK3CAmut, HR+, HER2- advanced breast cancer, intolerant to PI3Kα inhibitor3 (N = 15) RLY-2608 + Fulvestrant Arm PIK3CAmut, HR+ HER2- advanced / met BC MTD/RP2D Includes patients with non-measurable disease Registration path to be determined

RLY-2608 – Data Disclosure Part 1: Dose Escalation Demonstrate clinical proof of mechanism Part 2: Dose Expansion Establish early efficacy & confirm dose Part 3: Pivotal Trial Establish definitive efficacy RP2D Target Inhibition Tolerability Selectivity Early Signals of Efficacy Interpretable Efficacy Focus of Disclosure Focus of 1H 2023 disclosure: Acute safety and tolerability within context of mutant target inhibition

Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 Mutant + WT Tumor Agnostic SHP2 GDC-1971 Undisclosed 2 programs GD Genetic diseases 2 programs Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer

Clinical start anticipated in 2Q 2023 RLY-5836 – Similar Pre-clinical Profile, Different Chemical Properties from RLY-2608 Source: Internal RLY-5836 data 1. This model also carries a second mutation at K567R E545K mutant (MDAMB361) (mouse)1 H1047R mutant (HCC1954) (mouse) RLY-5836 achieved active doses with less insulin than orthosteric inhibitors

Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 Mutant + WT Tumor Agnostic SHP2 GDC-1971 Undisclosed 2 programs GD Genetic diseases 2 programs Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer

CDK2 – Highly Selective Inhibitors Identified Clinical start expected in early 2024 First compound synthesized to identification of lead compounds in <1 year RTX-1 RTX-2 Biochemical Potency CDK2/CycE IC50 (mM) 0.002 0.004 Biochemical Selectivity (fold over) CDK1/CycB 260x 100x CDK4/CycD1 685x 273x CDK6/CycD3 630x 322x CDK9/CycT1 3990x 2380x GSK3b 70250x 68050x Collect MD frames Extract features Cluster frames & assign cluster populations Predict selectivity Novel workflow leverages MD and ML to predict selectivity without bias or intervention Computational modeling enabled breakthrough speed Relay Tx’s CDK2 inhibitors observed to be highly selective Higher CDK2 activity associated with worse response to CDK4/6 inhibition in ER+ breast cancer CDK2 is important in ER+ breast cancer ~23K ~18K Patients receiving adjuvant CDK 4/6i Patients receiving 1L CDK 4/6i ~5K Patients receiving 2L CDK 4/6i Source: Internal analysis based on third party industry data

ERα Degrader – Rapidly Obtained Potent Compounds Development Candidate nomination expected in 2023 …to obtain potent ERα degraders Cellular proliferation Pathway suppression Relay Tx is leveraging rational design… Multiple experimental tools deployed to develop conformational models that enable effective triage of degrader design ideas Traditional Approach Relay Tx Approach Endocrine therapies are used in every line of therapy in HR+/HER2- Breast Cancer 195k annual US patients with HR+/HER2- breast cancer Second Line + First Line Adjuvant Line of Therapy Endocrine Tx Use of Endocrine Therapies Source: Internal analysis based on third party industry data

Relay Tx Solution – Highly Selective Breast Cancer Franchise Efficacy Target Inhibition Tolerability Selectivity Relay Tx Solution Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 Mutant + WT Preclinical Early Clin. Late Clin. …aims to address selectivity on validated targets for breast cancer The Relay Tx Solution…

Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 Mutant + WT Tumor Agnostic SHP2 GDC-1971 Undisclosed 2 programs GD Genetic diseases 2 programs Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer

SHP2 – Genentech Global Collaboration for GDC-1971 (Formerly RLY-1971) Collaboration provides meaningful economics to Relay Tx1 Source: World Lung 2022 #OA03.04 1. As of December 31, 2022: $105 million in upfront & milestone payments received, plus an opt-in option for 50/50 profit share and up to $690M in potential additional total milestones, low-to-mid teen royalties on global net sales plus eligible to receive additional royalties upon approval of GDC-1971 and GDC-6036 in combination GDC-1971 + Atezolizumab (PD-L1 Ab) initiated August 2022 GDC-1971 + GDC-6036 (KRAS G12Ci) initiated July 2021 Unconfirmed ORR: 53% (30/57 patients) Confirmed ORR: 46% (26/57 patients) Two ongoing trials with GDC-1971: Clinical Update for GDC-6036 Monotherapy at World Lung 2022

Target Program Breast Cancer PI3Kα franchise PI3KαPAN RLY-2608 RLY-5836 PI3KαSPECIFIC H1047R-specific Challengers CDK2 Selective CDK2 Challengers Degrader ERα Degrader Undisclosed 1 program FGFR2 RLY-4008 Mutant + WT Tumor Agnostic SHP2 GDC-1971 Undisclosed 2 programs GD Genetic diseases 2 programs Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer

Relay Tx – The Dynamo™ Platform 5+ Undisclosed Programs Inhibitors Degraders New Modalities Chaperones Platform capabilities and expertise continue to expand Enabling deep and diversified early pipeline

Target Program Annual US Patient # Breast Cancer1 PI3Kα franchise PI3KαPAN RLY-26082 ~8-51K ~50-156K all solid tumors RLY-58362 PI3KαSPECIFIC H1047R-specific ~4-25K ~15-48K all solid tumors Challengers CDK2 Selective CDK2 ~46K3 (Patients receiving CDK4/6i) Challengers Degrader ERα Degrader ~29-196K4 Undisclosed 1 program To be announced FGFR2 RLY-4008 Mutant + WT ~11-35K5 Tumor Agnostic SHP2 GDC-1971 ~37-69K6 Undisclosed 2 programs To be announced GD Genetic diseases 2 programs To be announced Relay Tx – Extensive Precision Medicine Pipeline Preclinical Early Clinical Late Clinical CCA + other Breast Cancer Note: Unless otherwise indicated, patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs 1. Unless otherwise indicated, all breast cancer patient numbers refer to HR+/HER2- breast cancer tumors 2. RLY-2608 covers H1047X, E542X, E545X hot spots, and breast cancer patient range assumes HR+/HER2- population 3. ~46k HR+/HER2- breast cancer patients expected to receive CDK 4/6 inhibitors in adjuvant setting, first-line setting, and second-line setting in 2023, per Decision Resources Breast Cancer Market Forecast, report dated June 2022 4. HR+/HER2- US late-line breast cancer patients compared to HR+/HER2- US incident breast cancer patients 5. FGFR2 altered late-line solid tumors compared to comprehensive annual FGFR2 altered incident solid tumors including additional FGFR gene fusions and rearrangements resulting from truncation of the protein at exon 18 6. SHP2 combo only includes KRAS G12C in lung and CRC, EGFR mutations in lung, and ALK fusions in lung

Relay Tx – Capital, Team & Execution Focus to Deliver $1.1B Cash, cash equivalents and investments as of the end of 3Q 2022 Current cash, cash equivalents and investments are expected to be sufficient to fund current operating plan into 2025 Tumor Agnostic Breast Cancer Franchise GDC-1971 (SHP2) RLY-4008 (Selective FGFR2) Clinical start in early 2024 Selective CDK2 Ongoing combo trials; Genentech controls data disclosures PI3KαPAN Development candidate nomination in 2023 ERα Degrader Full dose escalation data in 1H 2023 Non-CCA expansion cohorts data in 2H 2023 Pivotal cohort full enrollment in 2H 2023 Initial data in 1H 2023 To be announced 5+ undisclosed programs in preclinical development and additional early-stage efforts across platform Undisclosed RLY-2608 Clinical start in 2Q 2023 RLY-5836
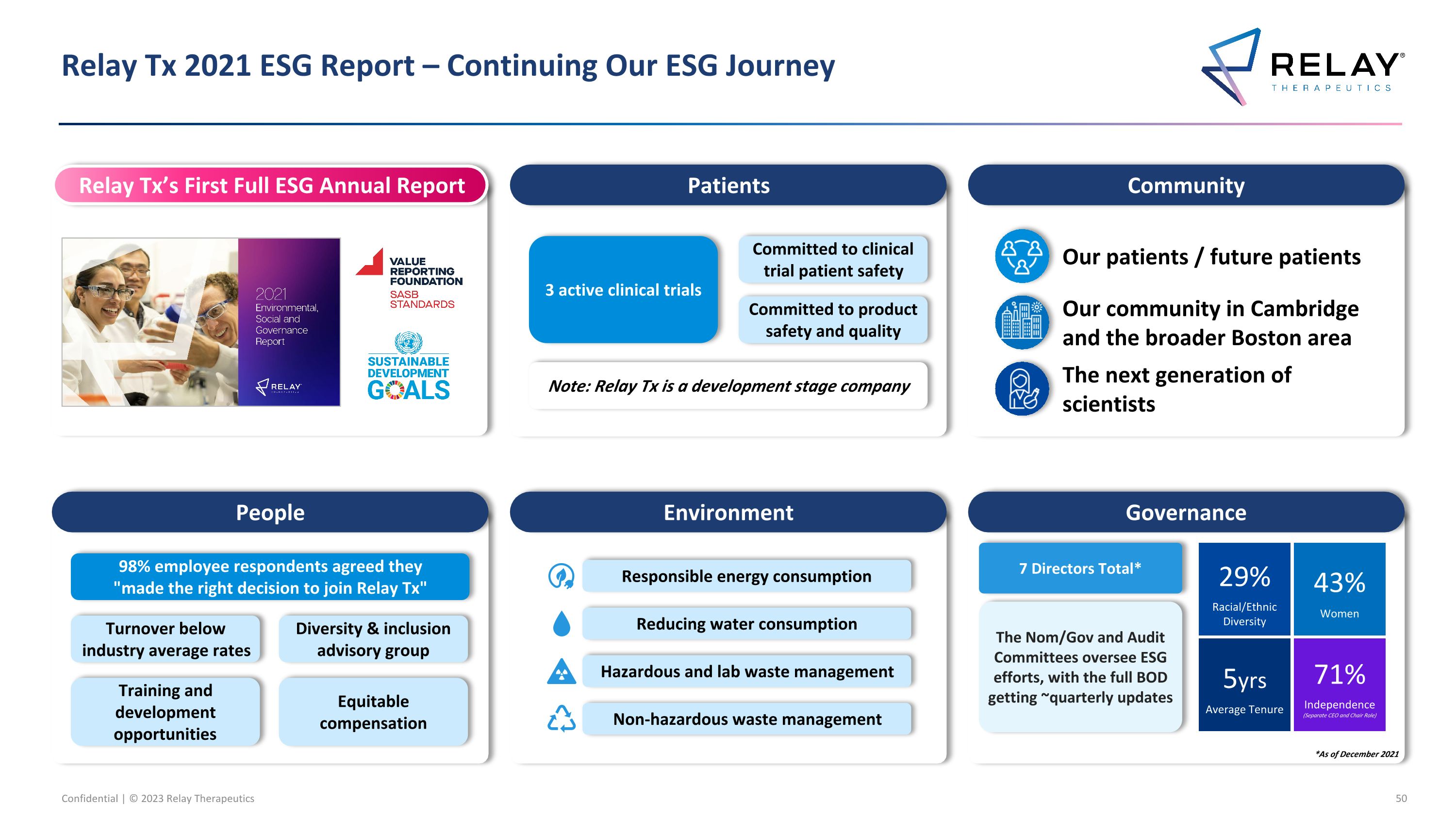
Relay Tx 2021 ESG Report – Continuing Our ESG Journey Relay Tx’s First Full ESG Annual Report Patients Community Environment Governance Our patients / future patients Our community in Cambridge and the broader Boston area The next generation of scientists *As of December 2021 People 98% employee respondents agreed they "made the right decision to join Relay Tx" Turnover below industry average rates Diversity & inclusion advisory group Training and development opportunities Equitable compensation 3 active clinical trials Committed to clinical trial patient safety Committed to product safety and quality Note: Relay Tx is a development stage company Responsible energy consumption Reducing water consumption Hazardous and lab waste management Non-hazardous waste management Average Tenure 5yrs Racial/Ethnic Diversity 29% Women 43% Independence (Separate CEO and Chair Role) 71% The Nom/Gov and Audit Committees oversee ESG efforts, with the full BOD getting ~quarterly updates 7 Directors Total*

